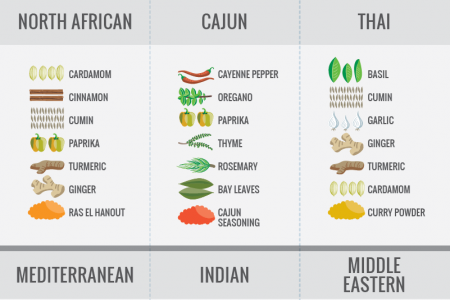
Maillard Reaction
MAILLARD REACTION reshuffling atoms, over heat, to make flavor molecules PROCESS flavor-full 70°F/21°C 212°F/100°C 250°F110°C > 300°F149°C 330°F/166°C 400°F/204°C (Maillard Reaction/browned) bland (steamed) sweet (caramelized) raw no taste inside (uncooked) <165°F (burned) More + more-varied proteins (meat vs. veggies) = more (stronger) flavors. Only the surface reaches the temperature at which the Maillard Reaction (discovered by chemist Louis Camille Maillard in the 1910s) can occur. browned onions (Maillard Reaction happens before caramelization) +heat flavors water (some of the) amino acids from protein sugars Heat and water causes hydrolysis or the breaking of the peptide bonds (in protein). Enzymes in your body perform this at lower temperatures and more efficiently. -Michael Klopfer AAAAA SAAAAAAA .н Sulfur IOANA Cen e MAILLARD REACTION reshuffling atoms, over heat, to make flavor molecules PROCESS flavor-full 70°F/21°C 212°F/100°C 250°F110°C > 300°F149°C 330°F/166°C 400°F/204°C (Maillard Reaction/browned) bland (steamed) sweet (caramelized) raw no taste inside (uncooked) <165°F (burned) More + more-varied proteins (meat vs. veggies) = more (stronger) flavors. Only the surface reaches the temperature at which the Maillard Reaction (discovered by chemist Louis Camille Maillard in the 1910s) can occur. browned onions (Maillard Reaction happens before caramelization) +heat flavors water (some of the) amino acids from protein sugars Heat and water causes hydrolysis or the breaking of the peptide bonds (in protein). Enzymes in your body perform this at lower temperatures and more efficiently. -Michael Klopfer Sulfur MAILLARD REACTION reshuffling atoms, over heat, to make flavor molecules PROCESS flavor-full 70°F/21°C 212°F/100°C 250°F110°C > 300°F149°C 330°F/166°C 400°F/204°C (Maillard Reaction/browned) bland (steamed) sweet (caramelized) raw no taste inside (uncooked) <165°F (burned) More + more-varied proteins (meat vs. veggies) = more (stronger) flavors. Only the surface reaches the temperature at which the Maillard Reaction (discovered by chemist Louis Camille Maillard in the 1910s) can occur. browned onions (Maillard Reaction happens before caramelization) +heat flavors water (some of the) amino acids from protein sugars Heat and water causes hydrolysis or the breaking of the peptide bonds (in protein). Enzymes in your body perform this at lower temperatures and more efficiently. -Michael Klopfer Sulfur MAILLARD REACTION reshuffling atoms, over heat, to make flavor molecules PROCESS flavor-full 70°F/21°C 212°F/100°C 250°F110°C > 300°F149°C 330°F/166°C 400°F/204°C (Maillard Reaction/browned) bland (steamed) sweet (caramelized) raw no taste inside (uncooked) <165°F (burned) More + more-varied proteins (meat vs. veggies) = more (stronger) flavors. Only the surface reaches the temperature at which the Maillard Reaction (discovered by chemist Louis Camille Maillard in the 1910s) can occur. browned onions (Maillard Reaction happens before caramelization) +heat flavors water (some of the) amino acids from protein sugars Heat and water causes hydrolysis or the breaking of the peptide bonds (in protein). Enzymes in your body perform this at lower temperatures and more efficiently. -Michael Klopfer Sulfur MAILLARD REACTION reshuffling atoms, over heat, to make flavor molecules PROCESS flavor-full 70°F/21°C 212°F/100°C 250°F110°C > 300°F149°C 330°F/166°C 400°F/204°C (Maillard Reaction/browned) bland (steamed) sweet (caramelized) raw no taste inside (uncooked) <165°F (burned) More + more-varied proteins (meat vs. veggies) = more (stronger) flavors. Only the surface reaches the temperature at which the Maillard Reaction (discovered by chemist Louis Camille Maillard in the 1910s) can occur. browned onions (Maillard Reaction happens before caramelization) +heat flavors water (some of the) amino acids from protein sugars Heat and water causes hydrolysis or the breaking of the peptide bonds (in protein). Enzymes in your body perform this at lower temperatures and more efficiently. -Michael Klopfer Sulfur MAILLARD REACTION reshuffling atoms, over heat, to make flavor molecules PROCESS flavor-full 70°F/21°C 212°F/100°C 250°F110°C > 300°F149°C 330°F/166°C 400°F/204°C (Maillard Reaction/browned) bland (steamed) sweet (caramelized) raw no taste inside (uncooked) <165°F (burned) More + more-varied proteins (meat vs. veggies) = more (stronger) flavors. Only the surface reaches the temperature at which the Maillard Reaction (discovered by chemist Louis Camille Maillard in the 1910s) can occur. browned onions (Maillard Reaction happens before caramelization) +heat flavors water (some of the) amino acids from protein sugars Heat and water causes hydrolysis or the breaking of the peptide bonds (in protein). Enzymes in your body perform this at lower temperatures and more efficiently. -Michael Klopfer Sulfur MAILLARD REACTION reshuffling atoms, over heat, to make flavor molecules PROCESS flavor-full 70°F/21°C 212°F/100°C 250°F110°C > 300°F149°C 330°F/166°C 400°F/204°C (Maillard Reaction/browned) bland (steamed) sweet (caramelized) raw no taste inside (uncooked) <165°F (burned) More + more-varied proteins (meat vs. veggies) = more (stronger) flavors. Only the surface reaches the temperature at which the Maillard Reaction (discovered by chemist Louis Camille Maillard in the 1910s) can occur. browned onions (Maillard Reaction happens before caramelization) +heat flavors water (some of the) amino acids from protein sugars Heat and water causes hydrolysis or the breaking of the peptide bonds (in protein). Enzymes in your body perform this at lower temperatures and more efficiently. -Michael Klopfer Sulfur
Maillard Reaction
Source
http://blog....action.jpgCategory
FoodGet a Quote