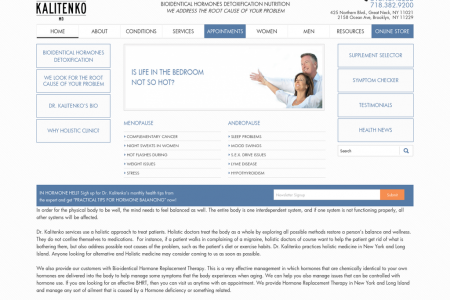
Parents Have Filed Hundreds of Zofran Lawsuits - How Did We Get Here?
PARENTS HAVE FILED HUNDREDS OF ZOFRAN LAWSUITS - How Did We Get Here?- 1983 Bendectin, America's only 1984 FDA approved morning sickness drug, is removed Hospitalizations for from the market. pregnancy related nausea and vomiting double. 1991 JANUARY 4 Zofran approved by the FDA to 1999 treat nausea and vomiting in cancer patients receiving certain types of chemotherapy. MARCH 9 FDA İssues GlaxoSmithKline a warning letter for distributing promotional materials that present Zofran In a manner that Is false or misleading because It lacks fair balance." 2001 O Zofran sales in the US reach $859 million. According to allegations eventually leveled by former employees Thomas Gerahty and Matthew Burke, GlaxoSmithKline begins to promote 2002 Zofran for offlabel use as a morning sickness treatment. Zofran sales in the US jump to $1.1 billion. 2003 Gerahty and Burke file their whistleblower lawsuit in the US District Court of Massachusetts. 2004 Einarson study, following the pregnancies of 176 women, finds 2006 no increase in major birth defects after Zofran ingestion. GlaxoSmithKline loses its patent on Zofran's active ingredient, ondansetron. DECEMBER 27 First generic versions of ondansetron approved by the FDA. 2009 Ondansetron overtakes prometha- zine as America's mostprescribed pharmaceutical treatment for morning sickness. 2012 JANUARY 4 Researchers at Harvard and Boston Univer- 2013 sity find 237% increase in cleft palate risk among babies exposed to Zofran during first trimester. FEBRUARY Pasternak study, revlewing 600,000 Danish pregnancies, finds no "statistically significant" link between Zofran and major birth defects. APRIL 8 Diclegis, a drug with same active ingredients as Bendectin, is approved by the FDA for "the treatment of nausea and vomiting of pregnancy." JUNE 29 FDA announces that ondansetron may adversely affect heart rhythms, possibly predisposing patlents to 'an abnormal and potentlally fatal heart rhythm known as Torsades de Pointes." JULY 2 Justice Department announces $3 billion settlement, resolving numerous claims against GlaxoSmithKline, Including allegations of Zofran's offlabel promotlon. The government's AUGUST lawsult was based on allegations first raised by Andersen study, revlewing over 900,000 Danish preg- Gerahty and Burke. nancles, finds bables exposed to Zofran during first trimester are 2 to 4 times more likely to have Twice as many pregnant women "hole In the heart" defects. are prescribed ondansetron as are prescribed promethazine, Zofran's main competitor in the morning sickness market. 2014 Dr. Gideon Koren notes that half of babies exposed to ondansetron in Pasternak study were exposed after 10 gestational weeks, ОСТОВЕR "when malformations could not be Danielsson study, reviewing more than 1 produced any more." million Swedish pregnancies, finds more than doubled risk of "hole in the heart" defects in bables exposed to ondansetron during first trimester. 2015 FEBRUARY 12 Minnesota mother files first Zofran lawsult, on behalf of two daughters, both born with JULY 6 congenital heart defects. Other GlaxoSmithKline asks Judicilal parents quickly follow. Panel on MultiDistrict Litigation to centralize Zofran lawsuits In Philadelphia. ОСТОВER 13 Judicial Panel on MultiDistrict Litigation issues court order, transferring 12 Zofran lawsuits to the US District Court of Massachusetts for DECEMBER 1 "consollidated pretrial proceedings." More than 150 Zofran lawsulits consolidated in US District Court of Massachusetts. DECEMBER 1 2016 GlaxoSmithKline files a motion to dismiss every Zofran lawsult. JANUARY 22 Judge F. Dennis Saylor IV rejects LAW GlaxoSmithKline's arguments for dismissal, allowing the lawsults to proceed. MARCH 15 238 Zofran lawsuits consolidated In Boston MDL. Thousands of families may still be eligible to pursue compensation. DESIGNED BY ZOFRANLEGAL.COM Pursuing Justice For Zofran Birth Defect Victims ••••
Parents Have Filed Hundreds of Zofran Lawsuits - How Did We Get Here?
Source
http://zofra...-lawsuits/Category
HealthGet a Quote