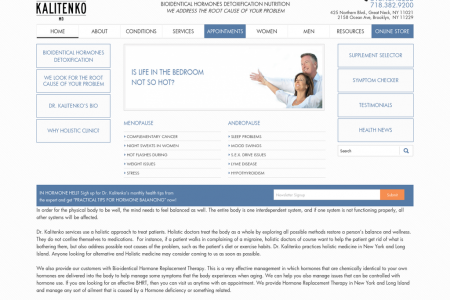
Vaccinating a Pandemic: The Hurdles Ahead for the Zika Virus
VACCINATING A PANDEMIC: THE HURDLES AHEAD FOR ZIKA As of February 2016, Zika has been declared a public health emergency by the World Health Organization. There are over 15 individual projects working to produce the first Zika vaccine to control the virus. With the Brazil outbreak starting in early 2015 and spreading to dozens of other countries, the world has taken notice. THE AVERAGE VACCINE TAKES 10 TO 15 YEARS TO FINALIZE, AND ZIKA IS ONLY AT THE BEGINNING OF THE PROCESS. THE STAGES OF VACCINE PRODUCTION Current STAGE 1 Zika Vaccine Stage Exploratory Stage (Duration: 2-4 years) Scientists work to identify natural or synthetic antigens that may prevent or treat a disease. Zika has spread to MORE THAN 20 COUNTRIES in South and Central America, and is present in Florida and the Gulf Coast in the United States. THE MAIN CONCERN It is believed that Zika is linked to microcephaly, a birth defect in which babies' brains don't fully develop, and they are born with abnormally small heads. At least 93 travel-related Zika cases are now documented in the U.S., in 22 states and Washington, D.C. WHAT SCIENTISTS KNOW 20 Zika is a flavivirus and shares the DEVELOPING A DENGUE VACCINE TOOK same DNA structure as dengue, also a mosquito-borne viral disease. YEARS STAGE 2 Preclinical Stage THIS IS ONLY THE FOURTH TIME A (Duration: 1-2 years) GLOBAL EMERGENCY HAS BEEN DECLARED SINCE 2007. Candidate vaccines are tested for safety using tissue or cell culture systems and animal testing. 2009 HIN1 swine flu outbreak STAGE 3 IND Application (Duration: 30 days) 2014 polio surge A sponsor submits an Investigational New Drug application to the FDA, summarizing the manufacturing and testing processes, lab reports, and proposed human clinical studies. 2014 Ebola epidemic 2015 Zika outbreak AS SOON AS THE IND APPLICATION IS APPROVED, CLINCAL TESTING ON HUMANS CAN BEGIN. PHASE 1 PHASE 2 PHASE 3 Small group tested (20-80 individuals) Several hundred Thousands of individuals tested individuals are tested FINAL Approved vaccines may go through phase IV trials, additional testing for safety, efficacy, and other potential uses. Approval and Licensure After a successful phase III trial, a Biologics License Application is submitted to the FDA. The FDA then inspects the factory where the vaccine will be made and approves labeling. DID YOU KNOW? Phase I of an Ebola vaccine started in September 2014, and there is still no final vaccine produced in the U.S. THE ROADBLOCKS TO A ZIKA VACCINE PREGNANT WOMEN AND CLINICAL TESTING Pregnant women are most at risk from infection of Zika, because the virus is linked to birth defects. However, researchers are debating whether vaccines and drugs developed for Zika would even be offered to pregnant women because of potentially harming the unborn. Because of the risks that come with developing vaccines and medications for pregnant women, the process to overcome potential regulatory hurdles would be lengthy. THE NEED FOR FUNDING When a disease is labeled as non-threatening, like Zika was until recently, there's little incentive for companies to fund research. EXTREMELY DIFFICULT TO DEVELOP Vaccines in general are very difficult to develop because creating a vaccine is about striking the perfect balance. Scientists need the vaccine to stimulate the immune system enough to produce antibodies without actually infecting the disease. VACCINE SWEET SPOT No vaccine issued. Disease continues to spread. Antibodies fail to rid body of antigens and patient is infected with Zika. Immune system stimulated and antibodies go to work without patient becoming infected. ISSUES WITH DISTRIBUTION Although it may be relatively simple to distribute vaccines to big cities, like Brazil, a lack of health facilities in remote areas poses a problem and makes it difficult to administer the vaccine in those areas, resulting in non-profit organizations needing to intervene. Also, there are strict regulations to guarantee safe transport. For example, if the vaccine were to fall outside the proper temperature ranges for too long, the vaccine could be declared invalid by the state health department. Proper Vaccine Temperature Range: 35°- 46°F DICKSON Sources: www.daytondailynews.com/news/news/local/zika-vaccine-could-take-months-years-to-develop/nqSXf www.wsj.com/articles/zika-vaccines-would-pose-special-risks-to-pregnant-women-1457309728 www.historyofvaccines.org/content/articles/vaccine-development-testing-and-regulation/ www.theatlantic.com/health/archive/2016/02/zika-yellow-fever-flaviviruses/462171/ www.cdc.gov/vaccines/pubs/pinkbook/downloads/vac-storage.pdf www.news-medical.net/health/Zika-vaccine-development.aspx www.ipha.ie/alist/vaccine-development-cycle.aspx www.who.int/medicines/emp_ebola_q_as/en/
Vaccinating a Pandemic: The Hurdles Ahead for the Zika Virus
Source
http://blog....ika-virus/Category
HealthGet a Quote