biomet-hip-lawyer-metal-on-metal-hip-replacement-side-effects-infographic
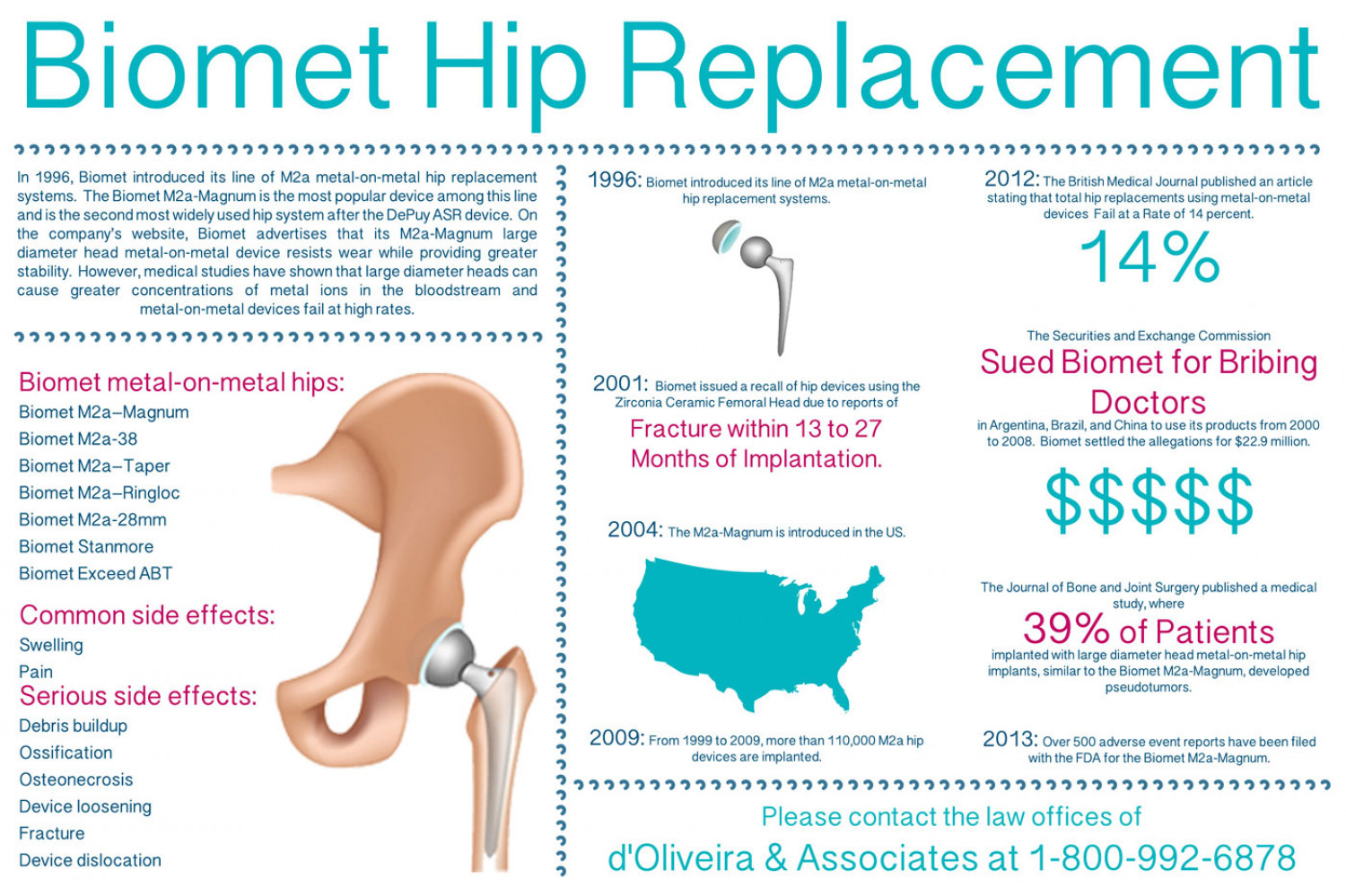
Biomet Hip Replacement 222222 222 222222222 22222222222222222222 In 1996, Biomet introduced its line of M2a metal-on-metal hip replacement systems. The Biomet M2a-Magnum is the most popular device among this line and is the second most widely used hip system after the DePuy ASR device. On the company's website, Biomet advertises that its M2a-Magnum large diameter head metal-on-metal device resists wear while providing greater stability. However, medical studies have shown that large diameter heads can cause greater concentrations of metal ions in the bloodstream and metal-on-metal devices fail at high rates. 2012: The British Medical Journal published an article 1996: Biomet introduced its line of M2a metal-on-metal hip replacement systems. stating that total hip replacements using metal-on-metal devices Fail at a Rate of 14 percent. 14% 22222222. 222222222 The Securities and Exchange Commission Sued Biomet for Bribing Biomet metal-on-metal hips: 2001: Biomet issued a recall of hip devices using the Zirconia Ceramic Femoral Head due to reports of Doctors Biomet M2a-Magnum Fracture within 13 to 27 in Argentina, Brazil, and China to use its products from 2000 to 2008. Biomet settled the allegations for $22.9 million. Biomet M2a-38 Biomet M2a-Taper Months of Implantation. $$$$$ Biomet M2a-Ringloc Biomet M2a-28mm 2004: The M2a-Magnum is introduced in the US. Biomet Stanmore Biomet Exceed ABT The Journal of Bone and Joint Surgery published a medical study, where Common side effects: 39% of Patients Swelling implanted with large diameter head metal-on-metal hip implants, similar to the Biomet M2a-Magnum, developed pseudotumors. Pain Serious side effects: Debris buildup 2009: From 1999 to 2009, more than 110,000 M2a hip devices are implanted. 2013: Over 500 adverse event reports have been filed Ossification with the FDA for the Biomet M2a-Magnum. Osteonecrosis 2222222: 22222222 Device loosening Please contact the law offices of Fracture d'Oliveira & Associates at 1-800-992-6878 Device dislocation
biomet-hip-lawyer-metal-on-metal-hip-replacement-side-effects-infographic
Source
http://www.g...raphic.jpgCategory
HealthGet a Quote